About the Disease – Multiple Myeloma (MM)
About the Disease – Multiple Myeloma (MM)
Multiple myeloma is caused by the dysregulated proliferation of plasma cells, characterized by the proliferation of monoclonal plasma cells that results in an overabundance of monoclonal immunoglobulin or its fragment (M protein). Clinical manifestations of MM include hypercalcemia, renal insufficiency, anemia, and the destruction of the bone. 1,2 MM primarily occurs in the elderly population. In the Chinese population, MM is the second most common hematologic malignancy, 3,4 with a median age of onset of 59 years1 and an incidence rate of 1.46 per 100,0004 that is lower than that of western countries. Although MM remains an incurable disease, the introduction of new therapies and diagnostic tools have led to significant advances in the clinical management of MM. The current standard of care therapies for MM include proteasome inhibitors, immunomodulatory agents, monoclonal antibodies and XPO1 inhibitors.1,2,5,6
- Account for 10% of all hematologic malignancies
- The second most common hematologic malignancy¹'³
- Account for 1% of all malignancies

therapies may turn it into a manageable chronic condition¹'²
1. Q&A about Multiple Myeloma 2020
2. Guidelines for the Diagnosis and Treatment of Multiple Myeloma (2022 revision)
3. Guan N, LI X, Wang S, et al. Incidence of Multiple Myeloma in Shenyang from 2009 to 2014. Cancer Research on Prevention and Treatment, 2019, 46(06): 547-550.
4. https://gco.iarc.fr/today/fact-sheets-populations
5. Wang D, Hao Y, Xiao M, et al. Epidemiology and etiology of multiple myeloma. Inter J Epidemionl Infect Dis, 2018,45(4): 277-280.
6. Version 5.2022,03/09/22 ⓒ 2021 National Comprehensive Cancer NetworkⓇ(NCCNⓇ)
 About the Disease – Diffuse Large B-Cell Lymphoma (DLBCL)
About the Disease – Diffuse Large B-Cell Lymphoma (DLBCL)
Diffuse large B-cell lymphoma (DLBCL) is one of the most common types of non-Hodgkin lymphoma (NHL) in adults and a highly heterogeneous malignancy in both clinical manifestations and prognosis. DLBCL accounts for 30%-40% of all NHL cases, and over 40% in most Asian countries. 1,2
The most common symptom of DLBCL is painless and progressive swelling of lymph nodes. Around 30% of patients with DLBCL experience B symptoms (fever, weight loss, and night sweats). Approximately 40% of DLBCLs originate from extranodal sites, and the most common extranodal sites are the gastrointestinal tract and bone marrow. 3
Currently, patients with DLBCL can achieve total remission after the first-line treatment with the R-CHOP regimen, with the other 40%-50% eventually relapsing or becoming refractory. Among those patients with relapsed/refractory DLBCL, approximately 10% can recover after standard salvage therapies and autologous hematopoietic stem cell transplantation, while the other 90% still face a poor prognosis. 4
2. Perry AM, Diebold J, Nathwani BN, et al. Non-Hodgkin lymphoma in the developing world: review of 4539 cases from the International Non-Hodgkin Lymphoma Classification Project. Haematologica. 2016;101(10):1244-1250.
3. Ansell SM. Non-Hodgkin lymphoma: diagnosis and treatment. Mayo Clin Proc. 2015;90(8):1152–1163
4. Clémentine Sarkozy, Laurie H. Sehn. New drugs for the management of relapsed or refractory diffuse large B-cell lymphoma. Ann Lymphoma 2019;3:10.
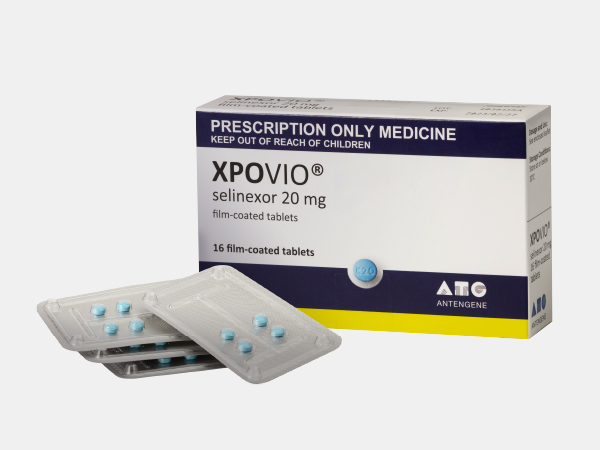
XPOVIO®(selinexor)
The world’s first oral selective inhibitor of the
nuclear export protein with a novel
mechanism
of
action

may have not been approved in all the countries where this website is accessible.
(MM) Mainland China
(DLBCL) South Korea Singapore Australia Taiwan,China HongKong,China Macau,China Malaysia Thailand Indonesia
Selinexor has been approved in a host of countries and regions including the U.S., the EU, Mainland China, Australia, and
Singapore. Antengene has the exclusive right to research, develop and commercialize selinexor in Greater China
(mainland China, Hong Kong, Taiwan, Macau), Australia, New Zealand, South Korea, and the ten ASEAN countries.
Product information stated in the National Medical Product Administration’s Database of Approved Drugs in China
| [Product Name] | |
Chinese generic name:塞利尼索片 Chinese brand name :希维奥® English generic name:Selinexor Tablets Generic name in Pinyin: Sailinisuo Pian | |
| [Indication]* | |
| In combination with dexamethasone for the treatment of adults with relapsed or refractory multiple myeloma who have received prior therapies including a proteasome inhibitor, an immunomodulatory agent and an anti-CD38 monoclonal antibody | |
| [Strength per Tablet] 20mg | |
[Packaging] 12 tablets/carton (3 tablets/blister, 4 blisters/carton), 16 tablets/carton (4 tablets/blister, 4 blisters/carton) | |
| [Shelf Life] 48 months | |
[Applicable Product Standard] Product standard for imported drugs JX20210104 | |
[Approval Number] NDA Approval Number HJ20210083 |
*XPOVIO (selinexor) Mainland China Product Information
Please see product insert for information related to dosing, adverse events, prohibited uses, warnings and precautions.
The information provided below is for healthcare professionals only, and the drug may have not been approved in all the countries where this website is accessible.
If you are a healthcare professional,
please click “Confirm” to proceed with the download
Otherwise please click “Cancel”

Product information stated in the National Medical Product Administration’s Database of Approved Drugs in China
| [Product Name] | |
Chinese generic name:塞利尼索片 Chinese brand name :希维奥® English generic name:Selinexor Tablets Generic name in Pinyin: Sailinisuo Pian | |
| [Indication]* | |
As a monotherapy for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who had received at least two lines of systemic therapy. | |
| [Strength per Tablet] 20mg | |
[Packaging] 12 tablets/carton, 16 tablets/carton, 20 tablets/carton, 32 tablets/carton | |
| [Shelf Life] 60 months | |
[Applicable Product Standard] Product standard for imported drugs JX20210104 | |
[Approval Number] NDA Approval Number HJ20210083 |
*XPOVIO (selinexor) Mainland China Product Information
Please see product insert for information related to dosing, adverse events, prohibited uses, warnings and precautions.
The information provided below is for healthcare professionals only, and the drug may have not been approved in all the countries where this website is accessible.
If you are a healthcare professional,
please click “Confirm” to proceed with the download
Otherwise please click “Cancel”

XPOVIO® (selinexor) ETC (Orphan drug)
Download the Product Insert
(for healthcare professionals only)| [The ingredients] | |
| 1tablet (167.2mg) | |
| [Active ingredient] | |
| selinexor∙∙∙ 20mg | |
| [Inactive ingredients] | |
| colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, Opadry 200 clear, Opadry II blue, povidone K30, and sodium lauryl sulfate [Appearance] blue, round, film‐coated tablets | |
| [Appearance] blue, round, film‐coated tablets | |
| [Indications And Usage]* | |
1. XPOVIO in combination with dexamethasone is indicated for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior therapies and whose disease is refractory to at least two proteasome inhibitors, at least two immunomodulatory agents, and an anti-CD38 monoclonal antibody. 2. XPOVIO is indicated the treatment of adult patients with relapsed or refractory diffuse large B‐cell lymphoma (DLBCL), not otherwise specified, including DLBCL arising from follicular lymphoma, after at least 2 lines of systemic therapy. |
*XPOVIO (selinexor) South Korea Product Information
Please see product insert for information related to dosing, adverse events, prohibited uses, warnings and precautions.
If you are a healthcare professional,
please click “Confirm” to proceed with the download
Otherwise please click “Cancel”

XPOVIO® (selinexor) ETC (Orphan drug)

Download the Product Insert
(for healthcare professionals only)XPOVIO (selinexor) Film Coated Tablet 20mg
FULL PRESCRIBING INFORMATION*
| [Multiple Myeloma] |
| • XPOVIO in combination with bortezomib and dexamethasone is indicated for the treatment of adult patients with multiple myeloma who have received at least one prior therapy. • XPOVIO in combination with dexamethasone is indicated for the treatment of adult patients with relapsed or refractory multiple myeloma who have received at least four prior therapies and whose disease is refractory to at least two proteasome inhibitors, at least two immunomodulatory agents, and an anti‐CD38 monoclonal antibody. |
| [Diffuse Large B‐Cell Lymphoma] |
| XPOVIO is indicated for the treatment of adult patients with relapsed or refractory diffuse large B‐cell lymphoma (DLBCL), not otherwise specified, including DLBCL arising from follicular lymphoma, after at least 2 lines of systemic therapy who are not eligible for haematopoietic cell transplant [see Clinical Studies (14.2)]. |
| [Dosage Forms And Strengths] |
|
Please see product insert for information related to dosing, adverse events, prohibited uses, warnings and precautions.
If you are a healthcare professional,
please click “Confirm” to proceed with the download
Otherwise please click “Cancel”

Selinexor 20 mg Oral Tablets
[NAME OF THE MEDICINE] Selinexor
[THERAPEUTIC INDICATIONS]*
XPOVIO is indicated:
•In combination with bortezomib and dexamethasone for the treatment of adult patients with multiple myeloma who have received at least one prior therapy.
•In combination with dexamethasone for the treatment of adult patients with relapsed or refractory multiple myeloma (RRMM) who have received at least three prior therapies and whose disease is refractory to at least one proteasome inhibitor, at least one immunomodulatory medicinal product (IMiD), and an anti-CD38 monoclonal antibody (mAb).
*XPOVIO (selinexor) Australian Product Information
If you are a healthcare professional,
please click “Confirm” to proceed with the download
Otherwise please click “Cancel”

Selinexor 20mg Film Coated Tablets
FULL PRESCRIBING INFORMATION*
[Multiple Myeloma]
•In combination with dexamethasone (Xd) for the treatment of adult patients withrelapsed/refractory multiple myeloma (R/R MM) who have received at least four prior therapies and whose disease is refractory to at least two proteasome inhibitors (PIs), at least two immunomodulatory agents (IMiDs), and an anti-CD38 monoclonal antibody.
•In combination with bortezomib and dexamethasone (XVd) for the treatment of adult patients with MM who have received at least one prior therapy.
[Diffuse Large B‐Cell Lymphoma]
as a monotherapy for the treatment of adult patients with relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL), not otherwise specified, including DLBCL arising from follicular lymphoma, after at least 2 lines of systemic therapy.
The fast-tracked approval for this indication is based on the tumor response rate (see section 14.2 for clinical studies). This indication still needs to undergo a confirmatory study in order to validate clinical benefits.
[Dosage Forms And Strengths]
Tablets: 20 mg, blue, round, bi‐convex, film‐coated tablets with “K20” debossed on one side and nothing on the other side.
* XPOVIO (selinexor) Taiwan Product Information
Please see product insert for information related to dosing, adverse events, prohibited uses, warnings and precautions.
If you are a healthcare professional,
please click “Confirm” to proceed with the download
Otherwise please click “Cancel”

XPOVIO Tablets 20mg
[Indications And Usage] *
[Multiple Myeloma]
• in combination with bortezomib and dexamethasone (XVd) for the treatment of adult patients with multiple myeloma (MM) who have received at least one prior therapy.
• in combination with dexamethasone for the treatment of MM in adult patients who have received at least four prior therapies and whose disease is refractory to at least two proteasome inhibitors, two immunomodulatory agents and an anti-CD38 monoclonal antibody, and who have demonstrated disease progression on the last therapy.
[Diffuse Large B‐Cell Lymphoma]
• for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), not otherwise specified, including DLBCL arising from follicular lymphoma, after at least 2 lines of systemic therapy who are not eligible for haematopoietic cell transplant.
[Pharmaceutical Form]
Film-coated tablet. Blue, round, bi-convex, film-coated tablet (4 mm thick and 7 mm in diameter) with “K20” debossed on one side.
* XPOVIO (selinexor) Hong Kong Product Information
Please see product insert for information related to dosing, adverse events, prohibited uses, warnings and precautions.
If you are a healthcare professional,
please click “Confirm” to proceed with the download
Otherwise please click “Cancel”

XPOVIO Tablets 20mg
[Indications And Usage] *
• in combination with dexamethasone for the treatment of multiple myeloma in adult patients who have received at least four prior therapies and whose disease is refractory to at least two proteasome inhibitors, two immunomodulatory agents and an anti-CD38 monoclonal antibody, and who have demonstrated disease progression on the last therapy.
[Pharmaceutical Form]
Film-coated tablet. Blue, round, bi-convex, film-coated tablet (4 mm thick and 7 mm in diameter) with “K20” debossed on one side.
* XPOVIO (selinexor) Macau Product Information
Please see product insert for information related to dosing, adverse events, prohibited uses, warnings and precautions.
If you are a healthcare professional,
please click “Confirm” to proceed with the download
Otherwise please click “Cancel”

XPOVIO 20 mg film-coated tablets
| [Therapeutic Indication]* | |
[Multiple Myeloma] • in combination with bortezomib and dexamethasone for the treatment of adult patients with multiple myeloma (MM) who have received at least one prior therapy. • in combination with dexamethasone for the treatment of MM in adult patients who have received at least four prior therapies and whose disease is refractory to at least two proteasome inhibitors, two immunomodulatory agents and an anti-CD38 monoclonal antibody, and who have demonstrated disease progression on the last therapy. [Diffuse Large B‐Cell Lymphoma] • for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) after at least 2 lines of systemic therapy, who are ineligible for autologous stem cell transplant. |
*XPOVIO (selinexor) Malaysia Product Information
Please see product insert for information related to dosing, adverse events, prohibited uses, warnings and precautions.
If you are a healthcare professional,
please click “Confirm” to proceed with the download
Otherwise please click “Cancel”

XPOVIO 20 mg film-coated tablets
| [Therapeutic Indication]* | |
• in combination with bortezomib and dexamethasone for the treatment of adult patients with multiple myeloma who have received at least one prior therapy. • in combination with dexamethasone for the treatment of multiple myeloma in adult patients who have received at least four prior therapies and whose disease is refractory to at least two proteasome inhibitors, two immunomodulatory agents and an anti-CD38 monoclonal antibody, and who have demonstrated disease progression on the last therapy. |
*XPOVIO (selinexor) Thailand Product Information
Please see product insert for information related to dosing, adverse events, prohibited uses, warnings and precautions.
If you are a healthcare professional,
please click “Confirm” to proceed with the download
Otherwise please click “Cancel”

XPOVIO film coated tablet 20 mg
[Multiple Myeloma]
•In combination with bortezomib and dexamethasone is indicated for the treatment of adult patients with multiple myeloma (MM) who have received at least one prior therapy.
•In combination with dexamethasone is indicated for the treatment of adult patients with relapsed/refractory multiple myeloma (R/R MM) who have received at least four prior therapies and whose disease is refractory to at least two proteasome inhibitors (PIs), at least two immunomodulatory agents (IMiDs), and an anti-CD38 monoclonal antibody.
[Diffuse Large B‐Cell Lymphoma]
•as a monotherapy for the treatment of adult patients with relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL), not otherwise specified, including DLBCL arising from follicular lymphoma, after at least 2 lines of systemic therapy who are not eligible for haematopoietic cell transplant.
*XPOVIO (selinexor) Indonesia Product Information
Please see product insert for information related to dosing, adverse events, prohibited uses, warnings and precautions.
If you are a healthcare professional,
please click “Confirm” to proceed with the download
Otherwise please click “Cancel”


XPOVIO® (selinexor) exerts
antitumor effects through three mechanistic pathways
Selinexor exerts antitumor effects by inducing the accumulation and activation of tumor suppressor proteins in the nucleus1, 4-7
Selinexor induces the accumulation of oncogenic mRNA in the nucleus, thus lowering the intracellular level of oncogenic proteins2
Selinexor restores the sensitivity to glucocorticoid by activating the glucocorticoid receptor pathway3
 Scientific Publications
Scientific Publications
{{item.article_des}}

 Learn More
Learn More
Report adverse events
Should you suspect any adverse events associated with our products, please contact us at:
-
Mainland China|Hotline: 400 821 7000Email: ae@antengene.com
-
South Korea|Hotline: 080 508 3243Email: safety.korea@antengene.com
-
Australia|Hotline: 1800 978 284
-
Taiwan,China|Hotline: (N/A)Email: safety.taiwan@antengene.com
-
For all other locations|Hotline: (N/A)Email: ae@antengene.com
Should you need any medical information
related to our products,
please contact us at:
-
Mainland China|Hotline: 400 821 7000Email: medinfo@antengene.com
-
South Korea|Hotline: 080 508 3243Email: medinfo.korea@antengene.com
-
Australia|Hotline: 1800 978 284Email: medinfo.au@antengene.com
-
HongKong,China|Hotline: (N/A)Email: medinfo.hk@antengene.com
-
Taiwan,China|Hotline: (N/A)Email: medinfo.tw@antengene.com
-
Singapore|Hotline: (N/A)Email: medinfo.sg@antengene.com
-
For all other locations|Hotline: (N/A)Email: medinfo@antengene.com
Since you have reported adverse events related to Antengene’s products, your/the patient’s information will be disclosed to Antengene’s pharmacovigilance (PV) department and entered into Antengene’s PV database which will also be reported to relevant authorities as required by related laws and regulations. Antengene may follow-up with you and/or the patient on the events you reported.
If you contact us via the hotline or E-mail, we will assume that you are aware of and consented to our collection and handling of your information. Your personal information will be strictly protected in accordance with Antengene’s privacy policies.